|
14.04.2018 13:00:00
|
ZEISS presents diagnostic and surgical advancements at ASCRS
WASHINGTON, April 14, 2018 /CNW/ -- The Medical Technology business group of ZEISS will present its latest ophthalmic diagnostic and surgical advancements at the 2018 American Society of Cataract and Refractive Surgery's Annual Meeting taking place April 13-17 in Washington, D.C. ZEISS will also be hosting three symposia and informative sessions with renowned experts throughout the event.
Integrated technologies for cataract surgeons to precisely1 align toric IOLs and improve refractive outcomes
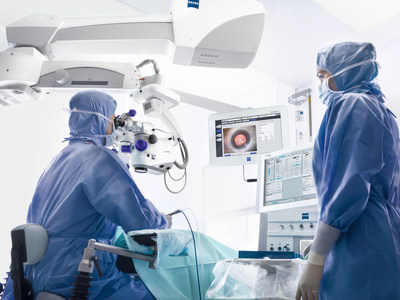
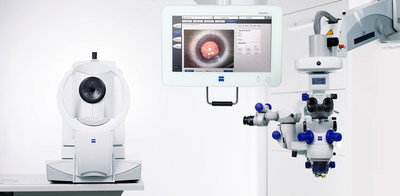
ZEISS will be presenting its complete portfolio of integrated diagnostic and surgical solutions that help cataract surgeons work more efficiently and deliver excellent outcomes for their patients. Using ZEISS Cataract Suite markerless, with the OPMI LUMERA® surgical microscope and CALLISTO® eye, the computer-assisted surgery system that enables the exchange of data between diagnostics and the OR, surgeons can more easily and precisely1 align toric IOLs, skipping the manual pre- and intra-operative marking steps and manual data transfer. IOLMaster® 700 Swept Source Biometry, when integrated into the toric IOL workflow of ZEISS Cataract Suite markerless, helps surgeons achieve target refraction and reduce the risk of refractive surprises for improved refractive outcomes for their patients.
Also on display during ASCRS will be VERACITY™ Surgical from ZEISS, an intuitive cloud-based platform for cataract surgery planning, logistics, treatment, risk management, and analysis. This latest digital connected eye care technology from ZEISS provides personalized technology-enabled patient care, synthesizing critical data at each step of the procedure to help cataract surgeons work more efficiently and reduce risks.
"We are continuing to expand our portfolio of integrated digital solutions that span from the office to the OR," says Jim Mazzo, Global President Ophthalmic Devices at Carl Zeiss Meditec. "Digitalization presents many opportunities to improve treatment outcomes and increase the efficiency of clinical workflows. Our goal is to continue to provide doctors and surgeons advanced technologies to help them care for their patients in the best way possible."
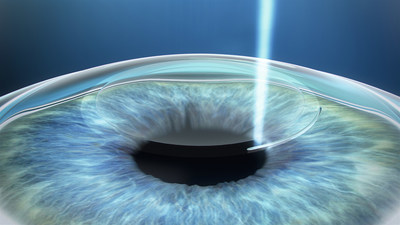
Surpassing milestone of 1M procedures worldwide, ZEISS celebrates one year of SMILE laser vision correction in the USA
ZEISS ReLEx® SMILE (Small Incision Lenticule Extraction) laser eye surgery is now being performed regularly around the world having been adopted by over 1300 surgeons in 69 countries, surpassing one million procedures worldwide. Using the VisuMax® femtosecond laser from ZEISS, SMILE provides refractive surgeons an additional proven laser vision correction option to offer their patients. A new clinical trial has begun outside the USA for SMILE in hyperopic patients. VisuMax Intracorneal Tunnel Cutting Option (ICR), which allows versatile incisions for the implantation of intracorneal rings, is now 510(k) cleared.
Professor Dan Z. Reinstein will be moderating a panel of experts to look back at the last year of SMILE at the ZEISS Meet the Experts session, "SMILE Adoption: Critical First Steps." He will also be available during the ASCRS to sign copies of his newly released book, "The Surgeon's Guide to SMILE: Small Incision Lenticule Extraction."
ZEISS Swept-Source OCT advancements pushing forward discovery in retinal research
Since its inauguration in 2016, the A R I Network, a global consortium of renowned doctors, clinicians, and scientists, has collaborated with ZEISS to advance the field of retinal imaging to drive further discovery and breakthroughs of new clinical applications for diseases affecting the retina. Having access to the latest Swept-Source OCT technology from ZEISS and with its Open Innovation approach, the A R I Network enables the exchange of ideas between members from around the world, facilitating collaboration to accelerate OCT development with ZEISS scientists. To date, the A R I Network has over 90 ongoing collaborative efforts to advance clinical practice and patient care, with over 45 publications and 60 posters.
"The A R I Network is a perfect example of how ZEISS collaborates with experts in their fields to develop new innovative solutions to advance patient care and to bring these technologies to everyday clinical use," says Dr. Ludwin Monz, President, and CEO of Carl Zeiss Meditec.
As a result of the A R I Network collaboration, new advanced technologies are being integrated into the PLEX® Elite 9000 Swept-Source OCT / OCTA platform from ZEISS to further doctors' understanding and analysis of the critical vasculature of the eye. Among these advancements include visualization tools enabling wider and deeper imaging of the eye for improved visualization of Age-related Macular Degeneration (AMD) and Diabetic Retinopathy. These new visualization technologies developed for PLEX Elite have been integrated into the CIRRUS® OCT and OCT Angiography platforms for use in daily clinical practice. Both instruments along with the recently launched ultra-widefield fundus imaging system, CLARUS™ 500, are part of ZEISS' comprehensive diagnostic portfolio.
For more information on ZEISS' scientific and educational program and events at ASCRS 2018: www.zeiss.com/ascrs.
1 Clinical data of Prof. Findl / Dr. Hirnschall presented at ESCRS 2013 – technically verified pre- / intraoperative matching precision ± 1.0° in mean.
Not all products, services or offers are approved or offered in every market and approved labeling and instructions may vary from one country to another. For country specific product information, see the appropriate country website. Product specifications are subject to change in design and scope of delivery as a result of ongoing technical development.
Brief profile Carl Zeiss Meditec:
Carl Zeiss Meditec AG (ISIN: DE 0005313704), which is listed on TecDAX of the German stock exchange, is one of the world's leading medical technology companies. The Company supplies innovative technologies and application-oriented solutions designed to help doctors improve the quality of life of their patients. The Company offers complete solutions, including implants and consumables, to diagnose and treat eye diseases. The Company creates innovative visualization solutions in the field of microsurgery. With approximately 3,000 employees worldwide, the Group generated revenue of €1,189.9m in financial year 2016/17 (to 30 September).
The Group's head office is located in Jena, Germany, and it has subsidiaries in Germany and abroad; more than 50 percent of its employees are based in the USA, Japan, Spain, and France. The Center for Application and Research (CARIn) in Bangalore, India and the Carl Zeiss Innovations Center for Research and Development in Shanghai, China, strengthen the Company's presence in these rapidly developing economies. Around 41 percent of Carl Zeiss Meditec AG's shares are in free float. The remaining approx. 59 percent are held by Carl Zeiss AG, one of the world's leading groups in the optical and optoelectronic industries. For more information visit our website at: www.zeiss.com/med.

![]() View original content with multimedia:http://www.prnewswire.com/news-releases/zeiss-presents-diagnostic-and-surgical-advancements-at-ascrs-300629667.html
View original content with multimedia:http://www.prnewswire.com/news-releases/zeiss-presents-diagnostic-and-surgical-advancements-at-ascrs-300629667.html
SOURCE Carl Zeiss Meditec
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!