|
25.02.2017 04:43:00
|
Endo Pharmaceuticals Inc. Issues Voluntary Nationwide Recall for One Lot of Edex® (alprostadil for injection) 10 mcg 2 Pack Carton Due to Potential Lack of Sterility Assurance
DUBLIN, Feb. 24, 2017 /PRNewswire/ -- Endo International plc (NASDAQ / TSX: ENDP) today announced that one of its operating companies, Endo Pharmaceuticals Inc. based in Malvern, Pennsylvania, is voluntarily recalling one lot of Edex® (alprostadil for injection) 10 mcg to the consumer level. This product recall is due to the detection by Endo of a defect in the crimp caps used in the manufacture of the subject product lot.
This defect has the potential to lead to a loss of container closure integrity, which could impact the product's sterility assurance and may lead to serious adverse events such as infections, both localized at the site of injection and systemically. To date, Endo has not received adverse event reports related to this recall.
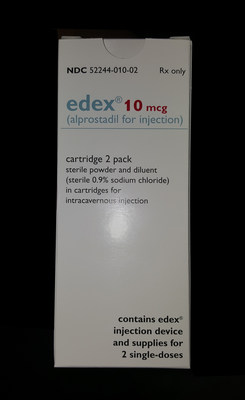
Edex® (alprostadil for injection) is a prescription only intracavernous injection indicated for the treatment of male erectile dysfunction. The recall applies to the 10 mcg strength, packaged in a 2 pack carton, (NDC 52244-010-02), product lot number 207386, Expiration Date: May 2019 (see photographs of packaged product within). The affected lot was distributed from December 13, 2016 through February 13, 2017 to wholesale distributors and retail pharmacies throughout the United States. The lot number can be found on the manufacturer's unit. Consumers who are unsure if they have the affected lot number should consult their pharmacist or health care professional.
Consumers in possession of any unused prescribed Edex® 10 mcg product bearing lot number 207386 should immediately discontinue use of the product and return the unused product by following the instructions below:
- Please contact Inmar at 1-844-529-1586, Monday through Friday (9am to 5pm EST) or email Edex@inmar.com for the following:
- Product Return
- Upon contacting Inmar and indicating you have unused product, please expect Return Authorization labels and Shipping instructions.
- Product Reimbursement
- Upon contacting Inmar, please be prepared to share proof of purchase.
- Proof of purchase can be sent to Edex@inmar.com or 635 Vine St. Winston Salem, NC 27101-Attention Recall Department, Edex Recall.
- Upon contacting Inmar, please be prepared to share proof of purchase.
- Product Return
Pharmacists and wholesalers are asked to check their inventories for lot number 207386, segregate any impacted inventory and contact Inmar at extension #1 at 1-800-967-5952, Monday through Friday (9 a.m. to 5 p.m. EST) or via e-mail at rxrecalls@inmar.com for instructions on product return. Pharmacists who have dispensed impacted product are asked to notify their patients of this recall. Pharmacies and wholesalers that received lot number 207386 will receive a letter as well as a copy of this press release with their recall notification information.
Endo takes this issue seriously and is fully committed to ensuring all of its products and packaging meet the highest quality standards. If you have any questions regarding this recall, please call 1-800-462-ENDO (3636), between the hours of 8:00 a.m. to 8:00 p.m. EST Monday through Thursday and 8:00 a.m. to 6:00 p.m. EST on Friday. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using this product. Additional information regarding this recall can be found at http://www.endo.com/endopharma/our-products.
Adverse reactions or quality problems associated with the use of this product may be reported to FDA's MedWatch Adverse Event Reporting program either by phone, on line, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
This Product Recall is being made with the knowledge of the United States Food and Drug Administration (FDA).
About Endo International plc
Endo International plc (NASDAQ / TSX: ENDP) is a highly focused generics and specialty branded pharmaceutical company delivering high-quality medicines to patients in need through excellence in development, manufacturing and commercialization. Endo has global headquarters in Dublin, Ireland, and U.S. headquarters in Malvern, PA. Learn more at www.endo.com.

To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/endo-pharmaceuticals-inc-issues-voluntary-nationwide-recall-for-one-lot-of-edex-alprostadil-for-injection-10-mcg-2-pack-carton-due-to-potential-lack-of-sterility-assurance-300413569.html
SOURCE Endo International plc
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!
Nachrichten zu Endo International plcmehr Nachrichten
| Keine Nachrichten verfügbar. |