|
02.08.2018 23:10:00
|
Boston Scientific Closes Acquisition of Claret Medical, Inc., Announces Positive Reimbursement Decision
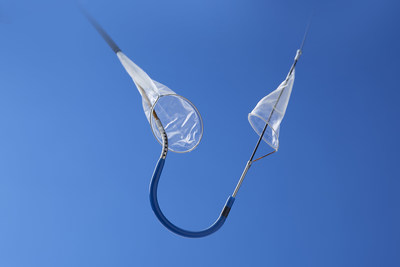
MARLBOROUGH, Mass., Aug. 2, 2018 /PRNewswire/ -- Boston Scientific Corporation (NYSE: BSX) announced it has recently closed its acquisition of Claret Medical, Inc., a privately-held company that has developed and commercialized the Sentinel® Cerebral Embolic Protection System, the only device cleared to protect patients against the risk of stroke in transcatheter aortic valve replacement (TAVR) procedures.
The company also announced that the U.S. Centers for Medicare and Medicaid Services (CMS) granted a New Technology Add-on Payment (NTAP) designation for the Sentinel System as part of the federal fiscal year 2019 Inpatient Prospective Payment System (IPPS). The NTAP designation, awarded to new medical devices determined to substantially improve the diagnosis or treatment of Medicare beneficiaries, will be effective on October 1, 2018.
"The Sentinel System is an exciting platform technology designed to reduce the risk of procedure-related stroke in TAVR and other left-heart and endovascular procedures, and is an increasingly important consideration for patients and physicians as the TAVR indication expands to treat a younger patient population," said Kevin Ballinger, president, Interventional Cardiology, Boston Scientific. "The recent CMS NTAP designation underscores the clinical value of the Sentinel System and will allow for accelerated adoption of this adjunctive therapy amongst structural heart centers."
The Sentinel System – which received CE Mark in 2014 and FDA clearance in 2017 – is the only device commercially available used to protect patients against the risk of stroke during TAVR, a minimally-invasive procedure to replace the aortic valve in patients with severe aortic stenosis. Embolic debris such as calcium or tissue can break loose during the procedure, travel through the bloodstream towards the brain and potentially cause neurological and neurocognitive damage. Recent studies have estimated approximately four percent of patients experience a clinically-apparent stroke within 30 days of a TAVR procedure.1,2,3,4,5,6 The landmark SENTINEL trial, which led to regulatory clearance, demonstrated that the Sentinel System reduced the incidence of strokes by 63 percent within the first 72 hours of the procedure.7
Boston Scientific announced a definitive agreement to acquire Claret Medical on July 20, 2018 for $220 million in up-front cash with an additional $50 million payment for reaching a reimbursement-based milestone, which has been fulfilled with the recent NTAP designation.
1 Leon, et al., N Engl J Med. 2010;363:1597-1607.
2 Webb, et al., J Am Coll Cardiol Intv. 2015;8:1797-1806.
3 Smith, et al., N Engl J Med. 2011;364:2187-98.
4 Leon, et al., N Engl J Med 2016;374:1609-20.
5 Popma, et al., J Am Coll Cardiol 2014;63:1972-81.
6 Adams, et al., N Engl J Med 2014;370:1790-98.
7 Kapadia S, et al. JACC. Jan 2017; 69(4): 367-377.
About Boston Scientific
Boston Scientific transforms lives through innovative medical solutions that improve the health of patients around the world. As a global medical technology leader for more than 35 years, we advance science for life by providing a broad range of high performance solutions that address unmet patient needs and reduce the cost of healthcare. For more information, visit www.bostonscientific.com and connect on Twitter and Facebook.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements may be identified by words like "anticipate," "expect," "project," "believe," "plan," "estimate," "intend" and similar words. These forward-looking statements are based on our beliefs, assumptions and estimates using information available to us at the time and are not intended to be guarantees of future events or performance. These forward-looking statements include, among other things, statements regarding the acquisition, the financial and business impact of the transaction, product launches and product performance and impact. If our underlying assumptions turn out to be incorrect, or if certain risks or uncertainties materialize, actual results could vary materially from the expectations and projections expressed or implied by our forward-looking statements. These factors, in some cases, have affected and in the future (together with other factors) could affect our ability to implement our business strategy and may cause actual results to differ materially from those contemplated by the statements expressed in this press release. As a result, readers are cautioned not to place undue reliance on any of our forward-looking statements.
Factors that may cause such differences include, among other things: future economic, competitive, reimbursement and regulatory conditions; new product introductions; demographic trends; the closing and integration of acquisitions; intellectual property; litigation; financial market conditions; and future business decisions made by us and our competitors. All of these factors are difficult or impossible to predict accurately and many of them are beyond our control. For a further list and description of these and other important risks and uncertainties that may affect our future operations, see Part I, Item 1A – Risk Factors in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission, which we may update in Part II, Item 1A – Risk Factors in Quarterly Reports on Form 10-Q we have filed or will file hereafter. We disclaim any intention or obligation to publicly update or revise any forward-looking statements to reflect any change in our expectations or in events, conditions or circumstances on which those expectations may be based, or that may affect the likelihood that actual results will differ from those contained in the forward-looking statements. This cautionary statement is applicable to all forward-looking statements contained in this document.
Use of Non-GAAP Financial Measures
To supplement our consolidated financial statements presented on a GAAP basis, we disclose certain non-GAAP financial measures, including adjusted net income and adjusted net income (earnings) per share that excludes certain charges and/or credits, such as amortization expense and acquisition-related net charges (credits). These non-GAAP financial measures are not in accordance with generally accepted accounting principles in the United States and should not be considered in isolation from or as a replacement for the most directly comparable GAAP financial measures. Further, other companies may calculate these non-GAAP financial measures differently than we do, which may limit the usefulness of those measures for comparative purposes. For further information regarding our non-GAAP measures, see Part II, Item 7 - Management's Discussion and Analysis of Financial Condition and Results of Operations in our most recent Annual Report on Form 10-K, which we may update in Quarterly Reports on Form 10-Q we have filed or will file hereafter.
CONTACTS:
Trish Backes
Media Relations
(651) 582-5887 (office)
trish.backes@bsci.com
Susie Lisa, CFA
Investor Relations
(508) 683-5565 (office)
BSXInvestorRelations@bsci.com
![]() View original content with multimedia:http://www.prnewswire.com/news-releases/boston-scientific-closes-acquisition-of-claret-medical-inc-announces-positive-reimbursement-decision-300691482.html
View original content with multimedia:http://www.prnewswire.com/news-releases/boston-scientific-closes-acquisition-of-claret-medical-inc-announces-positive-reimbursement-decision-300691482.html
SOURCE Boston Scientific Corporation
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!