|
17.06.2021 15:15:00
|
Ampio Provides Update on Osteoarthritis of the Knee (OAK) Program, Reiterates Compelling Data in Earlier Phase III Trials of Ampion in Severe OAK
ENGLEWOOD, Colo., June 17, 2021 /PRNewswire/ -- Ampio Pharmaceuticals (NYSE American: AMPE), a biopharmaceutical company focused on the advancement of immunology-based therapies for prevalent inflammatory conditions, today reported newly integrated data from four of its earlier clinical trials with consistent inclusion, exclusion, and demographic populations utilizing Ampion for treating osteoarthritis of the knee (OAK). Data from these 585 patients categorized as severe (i.e., Kellgren-Lawrence grade 4, or KL 4) demonstrates Ampion's consistent and statistically significant clinical effect in the reduction of pain compared to saline at two, ten, and twelve-week intervals subsequent to intra-articular injection.
"These 585 KL 4 patients in our earlier trials represent the largest available and reported dataset of severe OAK patients to date, and the data analysis clearly show Ampion as the first therapy to demonstrate clinical efficacy in patients suffering from severe OAK," said Michael Macaluso, President and CEO of Ampio. "Severe OAK remains a critical unmet medical need. We have already secured one pivotal trial that the FDA has agreed we can use to file our Biologics License Application (BLA), and, while our latest Phase III AP-013 clinical trial in OAK had to be paused because of the COVID-19 pandemic, the high degree of efficacy seen in the integrated data for KL 4 patients from the previous four trials gives us confidence to expect a positive outcome when it comes time to unblind this study."
Top-line results from Ampio's AP-003-A, AP-003-B, and AP-004 trials were previously published in Orthopedics (Orthopedics. 2018; 41(1):e77-e83), which reflected a clinically and statistically significant reduction in pain at weeks ten and twelve following a single injection, compared to saline, using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scale. In addition, Ampion showed a 35.6% and 33.2% reduction in pain at weeks ten and twelve compared to baseline, while saline showed a reduction of 29% and 24.7%, with a p value of 0.04 and 0.012 comparing the two, respectively.
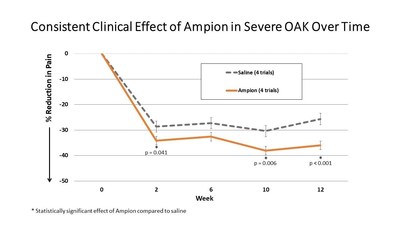
Today's announcement integrates data from severe OAK patients who were enrolled in Ampio's AP-003-C Phase III clinical trial with the previously published results. The pooled data show an even greater statistical significance, with a reduction in pain of 34.3% at two weeks (compared to 28.6% for saline, p value 0.041), 38.1% at ten weeks (30.4% for saline, p value 0.006) and 36% at twelve weeks (25.7% for saline, p value <0.001). See Figure 1.
Ampion has proven safe in thousands of enrolled and randomized patients, supporting repeat administration. No drug-related serious adverse events (SAEs) or treatment-related deaths have occurred, and the majority of adverse events (AEs) were unrelated to treatment. The incidence and severity of adverse events are similar for Ampion and saline, and the incidence of related AEs is far lower than that reported for existing therapies.
"Ampion is on track to become the first novel drug with unique mechanisms of action on the market for OAK in over 20 years. Ampion treatment has demonstrated consistent clinical efficacy in patients suffering from OAK across multiple trials, periods of time and clinical sites. In addition, the FDA has provided written confirmation that the AP-003-A study provides evidence of the effectiveness of Ampion" continued Macaluso. "We continue to remain confident the data from the suspended Phase III AP-013 trial will show similar safety and efficacy, but the decision whether to unblind this double masked, randomized controlled study or to continue adding patients ideally should be jointly decided with future potential partners."
About Osteoarthritis of the Knee (OAK)
According to a recent estimate published in The Lancet, around 650 million people worldwide suffer from osteoarthritis of the knee (OAK), an inflammatory, progressive, incurable disease characterized by elevated cytokines. Beyond being a mere quality of life issue, people who suffer from osteoarthritis have a significantly higher mortality rate than the general population.
Elevated levels of the cytokines TNFα and IL-12 are associated with pain and loss of function, while IL-1β and IL-6 are associated with disease severity. Ampion is the only therapy with demonstrated efficacy in severe OAK. Ampion's mechanism of action targets multiple pathways responsible for elevated cytokines to provide a therapeutic effect. Multiple human in vitro models have demonstrated the anti-inflammatory action of Ampion, reducing CXCL10 by up to 69%, IL-6 by up to 74%, IL-12 by up to 66% and TNFα by up to 55%.
About Ampio Pharmaceuticals
Ampio Pharmaceuticals, Inc. is a biopharmaceutical company primarily focused on the advancement of immunology-based therapies to treat prevalent inflammatory conditions for which there are limited treatment options. Ampio's lead drug, Ampion™, is backed by an extensive patent portfolio with intellectual property protection extending through 2035 and will be eligible for 12-year FDA market exclusivity upon approval as a novel biologic under the biologics price competition and innovation act (BPCIA).
Forward Looking Statements
Ampio's statements in this press release that are not historical fact, and that relate to future plans or events, are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by the use of words such as "believe," "expect," "plan," "anticipate," and similar expressions. These forward-looking statements include statements regarding Ampio's expectations with respect to Ampion and its classification, as well as those associated with regulatory approvals and other FDA decisions, the Biological License Application (BLA), the ability of Ampio to enter into partnering arrangements, clinical trials and decisions and changes in business conditions and similar events, the ability to receive regulatory approval to conduct clinical trials, that Ampion may be used to treat ARDS induced by COVID-19, all of which are inherently subject to various risks and uncertainties. The risks and uncertainties involved include those detailed from time to time in Ampio's filings with the Securities and Exchange Commission, including without limitation, under Ampio's Annual Report on Form 10-K and other documents filed with the Securities and Exchange Commission. Ampio undertakes no obligation to revise or update these forward-looking statements, whether as a result of new information, future events or otherwise.
Media Contact
Katie Kennedy
katie@gregoryfca.com
610-731-1045
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/ampio-provides-update-on-osteoarthritis-of-the-knee-oak-program-reiterates-compelling-data-in-earlier-phase-iii-trials-of-ampion-in-severe-oak-301314744.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/ampio-provides-update-on-osteoarthritis-of-the-knee-oak-program-reiterates-compelling-data-in-earlier-phase-iii-trials-of-ampion-in-severe-oak-301314744.html
SOURCE Ampio Pharmaceuticals, Inc.
 Der finanzen.at Ratgeber für Aktien!
Der finanzen.at Ratgeber für Aktien!
Wenn Sie mehr über das Thema Aktien erfahren wollen, finden Sie in unserem Ratgeber viele interessante Artikel dazu!
Jetzt informieren!
Nachrichten zu Ampio Pharmaceuticals Incmehr Nachrichten
| Keine Nachrichten verfügbar. |